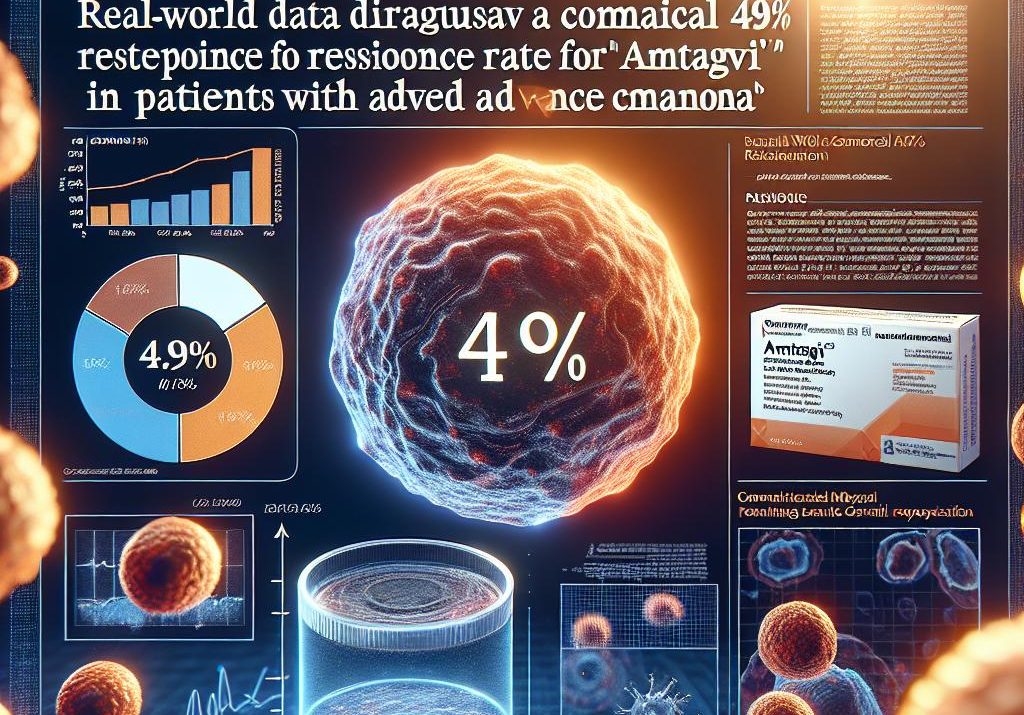
Iovance Biotherapeutics’ real-world data reinforces the clinical value of Lifileucel (amtagvi) in advanced melanoma. The recently released data from four authorized treatment centers shows a 48.8% objective response rate (ORR) among 41 evaluable patients previously treated with immune checkpoint inhibitors (ICIs), and potentially targeted therapy. Notably, the ORR climbed to 60.9% for patients receiving Lifileucel as a third-line or earlier treatment. This highlights the potential for improved outcomes when Lifileucel is incorporated earlier in the treatment sequence.
This real-world evidence adds another layer of validation to Lifileucel’s position in the advanced melanoma treatment landscape. The data arrives at a critical juncture as the field continues to grapple with optimizing treatment sequencing and maximizing patient outcomes in this challenging disease. While accelerated approval was granted based on ORR and duration of response in the C-144-01 trial, demonstrating efficacy in real-world settings is crucial for cementing market acceptance and influencing payer coverage decisions.
The higher response rate observed in less heavily pretreated patients raises important strategic questions for both Commercial and Medical Affairs teams. For Commercial teams, this data provides a compelling argument for earlier intervention with Lifileucel. It allows them to position the therapy not just as a salvage option but as a potential cornerstone of treatment after ICI failure. This shift has significant implications for market access strategies, particularly in securing favorable formulary placement.
For Medical Affairs, the real-world data provides a springboard for more robust HCP engagement. Generating and disseminating this type of evidence becomes critical for educating oncologists about the potential benefits of earlier Lifileucel utilization. This data can also inform the design of future real-world studies, including comparative effectiveness research, to further strengthen Lifileucel’s value proposition.
Looking ahead, the continued generation of real-world evidence will be pivotal in determining Lifileucel’s long-term market success. While these initial results are promising, ongoing data collection and analysis, including longer-term follow-up and exploration of patient subgroups, will be essential. Furthermore, the ongoing Tilvance-301 phase 3 trial evaluating Lifileucel in the frontline setting for advanced melanoma will be a critical inflection point, potentially expanding the therapy’s reach and market potential. The ability to seamlessly integrate this upcoming data with existing real-world evidence will be crucial for Iovance to solidify Lifileucel’s role in the evolving treatment paradigm for advanced melanoma.
Jon Napitupulu is Director of Media Relations at The Clinical Trial Vanguard. Jon, a computer data scientist, focuses on the latest clinical trial industry news and trends.